KeraMed News Room
First KeraKlear surgeries performed in the Kingdom of Saudi Arabia by Dr. Raed Alomair
On September 13, 2022 Dr. Raed Alomair of Elite Hospital in Riyadh performed the first KeraKlear surgery in the Kingdom of Saudi Arabia. Using the…
Read More
Tibbetts Award Recipient
KeraMed, Inc. is the recipient of the SBA Tibbetts award. The Tibbetts Award honors the SBIR/STTR program participants and supporters that have created a significant…
Read More
KeraMed Videos

Dr. Kanellopoulos’ interview in Barcelona for Keratoprosthesis
In this video Dr. William F. Wiley interviews Dr. Kanellopoulos about the benefits of using the KeraKlear Artificial Cornea. Dr. Kanellopoulos discusses the benefits of…
Watch Video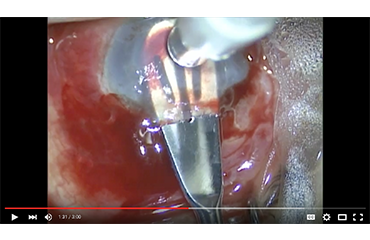
KeraKlear Implantation Technique by Dr. Kanellopoulos
Keraklear Inlay keratoprosthesis in an aniridia keratopathy and glaucoma case. Dr. Kanellopoulos discusses the impantation of the KeraKlear using an intrastromal pocket at 300um using…
Watch Video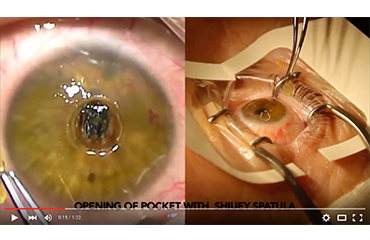
KeraKlear Artificial Cornea Implantation by Kashif Baig, MD
KeraKlear Artificial Cornea implantation procedure for the treatment of keratoconus after crosslinking. In this video Dr. Baig demonstrates the opening of the pocket using the…
Watch Video
KeraKlear Artificial Cornea
The KeraKlear is a revolutionary new type of foldable artificial cornea that is designed for synthetic lamellar keratoplasty through small incisions.
The KeraKlear is now available for treatment of all of the most common forms of cornea blindness including: keratoconus, corneal scars, corneal dystrophies, limbal stem cell deficiency, corneal edema and failed corneal transplants.
Can be implanted at depths from 300 microns to 700 microns to replace opacified or edematous corneal tissue.
Learn more about the KeraKlearKeraMed Products
KeraMed Inc. is an ISO13485:2016 and CE Mark certified company that specializes in devices for minimally invasive corneal surgery. Each device has been designed to treat corneal conditions using smaller incisions than ever before possible. Smaller incisions can enable faster surgery, faster patient recovery, and maintain the structural strength of the cornea.
Product Disclaimer
CAUTION: The KeraKlear Artificial Cornea is an investigational device, limited by federal (U.S.) law to investigational use.











