KeraKlear Non-Penetrating Artificial Cornea
The KeraKlear Artificial Cornea is a medical device for the treatment of cornea blindness. The KeraKlear is designed to be implanted into a partial thickness corneal pocket made by a femtosecond laser through an opening of 3.5 mm in diameter.
The KeraKlear is indicated for patients that have non-inflammatory forms of corneal blindness (e.g. keratoconus, corneal dystrophies, corneal scars, and corneal edema). The KeraKlear should not be used in patients with inflammatory causes of corneal blindness such as Steven's Johnson Syndrome, ocular cicatricial pemphigoid or atopic keratoconjunctivitis.
- In patients with non-inflammatory corneal blindness and corneal opacity the KeraKlear improves vision in > 90% of cases
- In patients with non-inflammatory corneal blindness and corneal opacity there is a high retention rate >90% after mean follow-up of 43 months
- KeraKlear implantation does not penetrate the anterior chamber and removes less tissue than either penetrating keratoplasty or deep anterior lamellar keratoplasty
- KeraKlear has a lower rate of glaucoma than Boston Kpro and keratoplasty and a lower rate of endophthalmitis than Boston Kpro.
- KeraKlear XT can be stocked in ambient conditions so there is no need to wait for donor tissue.
- Significantly faster procedure than penetrating keratoplasty. KeraKlear Implantation is typically 15 to 20 minutes, whereas penetrating keratoplasty is typically 1 to 2 hours
Below is an example of a patient who underwent KeraKlear implantation in Venezuela by Dr. Jose Vargas. This patient has a history of two previous failed corneal transplants due to graft rejection, the last one having been performed 2 years prior to KeraKlear surgery. Pre-operatively, this patient had hand motion vision only.
Figure 1. - Pre-Op
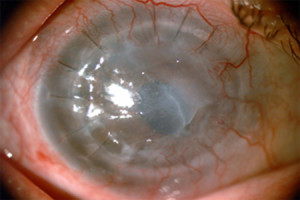
Fig. 1. Shows the pre-operative appearance of the patient's eye. Note the severe opacification and irregularity of the failed cornea graft which is due to corneal edema.
Figure 2. - Post-Op
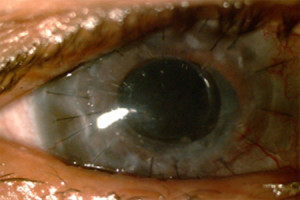
Fig. 2 shows the post-operative appearance of the patient's eye. Note how the central cornea is clear after the KeraKlear has been implanted. At one month the patient's vision improved to 20/60 without correction and has remained stable through 12 months. This level of visual acuity is sufficient to obtain a driver's license.
Figure 3. - Iris Details Visible through KeraKlear
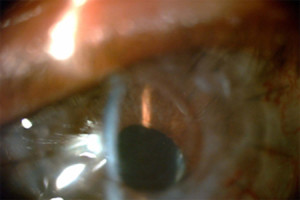
Fig. 3 shows a high resolution view which shows how the iris details are now visible through the KeraKlear and the patient's failed graft.
Figure 4. - View of OpticNerve through slitlamp
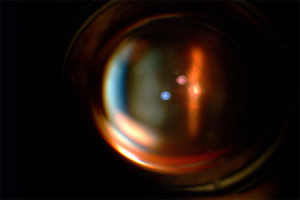
Fig. 4. Shows a slit-lamp photograph of the optic nerve through the KeraKlear, which demonstrates the ability to visualize the fundus and a clear optical pathway.
The success of the KeraKlear for treating multiple corneal transplant failures gives hope that a minimally invasive surgery may become available for this important group of cornea blind patients. This case also demonstrates the feasibility of the KeraKlear to improve the vision in the setting of cornea endothelial dysfunction.
Instructions for Use:
If a printed copy is desired, please print a copy from the links above. Alternatively, you may request a printed version of the pdf document from the distributor of the KeraKlear in your country.

